Safety Monitoring at the Core of Your Priorities
Ensuring product safety at every stage of their lifecycle is essential to protect public health and preserve your company’s reputation.


Your Goals, Our Priorities
- Bring safe products to market quickly and protect public health
- Reduce health and commercial risks linked to product use and maintain trust among healthcare professionals and patients
- Ensure regulatory compliance of your products
- Manage your internal resources and expertise by outsourcing to trusted partners so you can focus on innovation and performance in the health sector
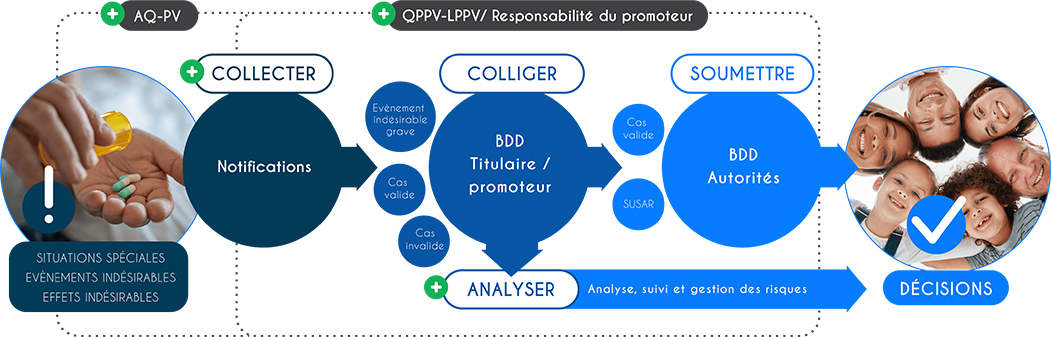
Gérer les cas Vigilances, de la réception à la soumission, anticiper, maitriser et prévenir les risques du portefeuille produit, mettre en place et maintenir un système de Vigilances efficient et compliant, maintenir un système qualité garantissant la pérennité du système de gestion de Vigilances, vous améliorer sur une gestion ultra performante, consultez nos expertises en pharmacovigilance.
Regulatory Expertise to Support Your Products
Regulatory Affairs are central to the responsibilities of pharmaceutical laboratories. Managing this function thoroughly is essential for securing the product lifecycle, from development to market authorization and beyond.
Our Expertise by Your Side, Every Step of the Way
Prepare, obtain, update: each stage of market authorization (MA) requires rigor, expertise, and foresight.
- Ensure product compliance with health authority requirements
- Accelerate market access by optimizing registration processes
- Avoid non-compliance risks and regulatory penalties
- Optimize product lifecycle management to adapt to regulatory changes
Medical Information: Responsiveness, Reliability, Expertise
- Handling of medical information requests
- Support for healthcare professionals and patients
- Traceability and analysis of medical data
- Integration of requests into pharmacovigilance and quality systems
- Optimization of medical knowledge databases



Compliance or Quality Audit
Optimize your processes, secure your practices, ensure your compliance: an independent audit is essential for evaluating, adjusting, and anticipating.
- Ensure regulatory compliance with international standards
- Improve industrial process quality and enhance operational performance
- Manage risks and prevent non-conformities that could impact patient safety
- Strengthen trust with health authorities, partners, and clients
- Effectively prepare for regulatory inspections and meet certification body requirements
Quality: Ensuring Excellence from Development to Delivery
- Ensure patient safety by delivering compliant and reliable products
- Comply with international standards and regulations (GMP, GDP, ISO, ICH, FDA, EMA)
- Prevent risks and non-conformities that could lead to product recalls
- Optimize traceability and process management for long-term performance
- Prepare for audits and inspections by health authorities


Transparency in Healthcare
- Declaration or prior authorization request for certain benefits (invitations, payments, donations…)
- Submission of contracts and agreements to the appropriate authorities (Medical Councils, Regional Health Agencies, etc.)
- Transparent publication of conflicts of interest on the government platform Transparence-Santé
Training
- Deliver high-quality training tailored to roles and skill levels
- Provide flexible and effective educational tools
- Strengthen team skills, certification, and operational impact
- Train key stakeholders in dispensing, including pharmacists and pharmacy staff
Promoting a Product Begins with Communication
- Strengthen brand and product awareness
- Improve visibility in pharmacies and among distributors
- Drive consistent pharmacist ordering
- Adapt to new health communication practices

Transition Managers
Is your company undergoing a strategic shift, facing a crisis, or managing the departure of a key leader? What if a transition manager could immediately step in to ensure continuity and deliver tangible results?
