At Universal Medica Group, patient safety and effective risk management related to healthcare products are our highest priorities.
For over 20 years, we have supported life sciences companies with the compliant and thorough management of their pharmacovigilance and regulatory safety responsibilities.
With recognized expertise and advanced tools, we provide fully tailored solutions to meet global regulatory requirements and ensure patient safety throughout the entire product lifecycle.


Comprehensive Expertise in Health Product Vigilance
Vigilance is central to the safety of healthcare products. Our specialized teams handle all areas of health product vigilance :
- Pharmacovigilance: Monitoring adverse drug reactions (originator drugs, generics, biosimilars)
- Materiovigilance: Tracking incidents related to medical devices
- Cosmetovigilance: Monitoring adverse effects associated with cosmetic products
- Nutrivigilance: Managing reports involving dietary supplements
- Toxicovigilance: Monitoring toxic risks from exposure to specific substances
Why is this comprehensive approach essential?
By integrating all vigilance areas, we ensure consistent and thorough risk management for your products, safeguarding patient safety and maintaining regulatory compliance.
Our Pharmacovigilance Services: Comprehensive Support
Our specialized teams offer a range of services tailored to the needs of pharmaceutical companies and healthcare manufacturers.
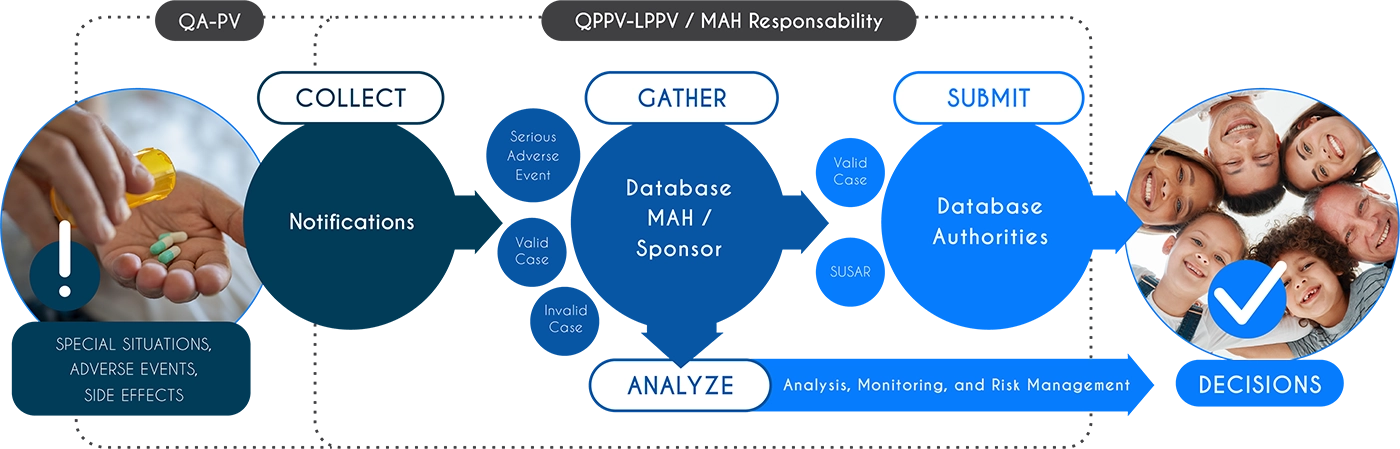
Pharmacovigilance Case Management
- Receipt and analysis of spontaneous reports (from patients, healthcare professionals, authorities)
- Collection and documentation of cases from clinical trials and post-marketing studies
- Entry of cases into validated databases (PV E2B R3)
- Medical evaluation, causality assessment, and identification of serious and unexpected adverse events
- Preparation and submission of reports to health authorities in compliance with regulatory timelines
Case Study : Our teams recently integrated over 4,000 pharmacovigilance cases in record time during the acquisition of a product portfolio by a partner laboratory.
Universal Medica Group for daily and rigorous support in Vigilance matters.
Priority is given to patient safety in all countries and to both local and international regulatory excellence.
Risk Detection and Proactive Management
- Proactive monitoring of safety signals using specialized tools (EVDAS, scientific databases, etc.)
- Implementation of Risk Management Plans (RMPs) and Additional Risk Minimization Measures (ARMMs)
- Development and dissemination of safety communications for healthcare professionals and patients
Our added value: A proactive approach that helps prevent critical situations and ensures optimal responsiveness to risk signals.


Preparation and Submission of Regulatory Reports
- Preparation and management of PSURs, DSURs, PBRERs, ASRs et PSUSAs
- Drafting of DMIs (Information Modification Requests)
- Preparation and updating of the Pharmacovigilance System Master File (PSMF)
- Management of pharmacovigilance-related inspections and audits
Client testimonial: « The responsiveness and quality of your teams during our recent ANSM inspection enabled us to renew our marketing authorization without any critical observations. Many thanks to your teams for their thoroughness and professionalism. »
Implementation and Management of High-Performance Vigilance Systems
- Deployment and configuration of databases compliant with international standards (PV E2B R3)
- Integration and migration of historical cases to new databases
- Rigorous quality monitoring of processes to ensure compliance and traceability of actions
Support and Training in Pharmacovigilance
- Provision of QPPV (Qualified Person for Pharmacovigilance) and LPPV (Local Person for Pharmacovigilance) to manage your local and European regulatory responsibilities
- Development of internal procedures and training of teams on pharmacovigilance best practices
- Delivery of training on the principles and regulatory requirements of vigilance activities
Our strength: Our training sessions are conducted by subject-matter experts and are available either in person or via our e-learning platform, EuroAcademy.
Expertise Supporting Your Compliance and Performance
Our teams have deep knowledge of the requirements set by major health authorities, including:
- EMA (European Medicines Agency)
- FDA (Food and Drug Administration)
- ANSM (French National Agency for the Safety of Medicines and Health Products)
- MHRA, Swissmedic, and other international authorities
Our expertise ensures:
- Rigorous and compliant management of your regulatory obligations
- Significant reduction in non-compliance risks
- Optimization of your processes to enhance efficiency and peace of mind


Why Choose Universal Medica Group for Your Pharmacovigilance Activities?
- Proven Experience: Over 20 years of expertise serving the healthcare and life sciences industries
- Dedicated Team: Experts trained in the latest regulations and methodologies
- Advanced Tools: Cutting-edge technology solutions for efficient and secure case management
- Proactive Approach: Risk anticipation to protect patient safety
- Flexibility and Adaptation: Custom solutions tailored to your specific needs
Universal Medica Group: Your Trusted Partner in Pharmacovigilance
By entrusting us with your pharmacovigilance activities, you gain a reliable and expert partner, capable of managing all your regulatory responsibilities with rigor and responsiveness.
Want to learn more? Contact our team today to find out how we can support your pharmacovigilance and health vigilance projects.
